Long time no post. I have been busy with other projects. Over the weekend I tried aqua regia assaying some of the rocks I have collected.
Here are photos of sample rocks I assayed,
Sample 1
View attachment 1621206
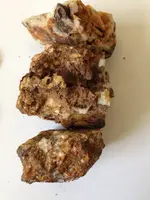
Sample 2
View attachment 1621207
Sample 3
View attachment 1621209
Sample 4
View attachment 1621210
Sample 5
View attachment 1621211
Assay procedure:
This is credit to Deano from Goldrefiningforum.com, first rocks are pulverized, sifted to fine powder, mixed up well, and a 20 gram sample is collected.
In a 600 ml beaker, 250ml of 4:1 aqua regia is prepared (that is 200ml hydrochloride acid mixed with 50ml nitric acid).
20 gram sample is added slowly to the aqua regia solution and let it simmer for 20-30 minutes. First you notice the brown fumes of nitric and as process closes to end the white fumes appear, let it simmer for another 5 minutes and cool it off to room temperature.
Now take a drop from aqua regia solution and add a drop of stannous chloride testing solution, if color of solution changed to a pale purple to purple, it indicates there was gold in your sample.
I went through all samples using this procedure, and only sample 5 turned to a pale purple indicating gold in it. The rest of samples were negative.
Here is procedure for sample 4 for your reference,
Sample 4 pulverized and sifted,
View attachment 1621222
The sample is added to warn aqua regia acid in a glass jar in water bath,
View attachment 1621223
Brown fumes emerge as ore sample dissolves,
View attachment 1621224
This is the end of aqua regia dissolution,
View attachment 1621225
Here is photo of sample 4 aqua regia solution after it is cool,
View attachment 1621226
If there is any silver in ore sample, it can be detected as purpule layer of silver chloride on top of the ore at the bottom if you had left the beaker outside in the Sun to cool.
This is a drop of the solution,
View attachment 1621227
Addition of a drop of stannous chloride test solution to above yield no color change,
View attachment 1621230
So it means no gold was in the sample 4. The rest of them turned out to be like this, except the sample 5 as it can be seen here,
A drop of sample 5 aqua regia solution,
View attachment 1621232
Testing that with a drop of stannous chloride test solution,
View attachment 1621233
Hope these help you out there prospecting, it is very cheap compared to other methods, and can tell if any rocks has gold or not. There is a colorimetric method to actually assay the quantity of gold in a sample using aqua regia assaying which I will post about it.
Next step would be a cyanide leach test.
Thanks and best regards
Kj