Goes4ever
Silver Member
- Jan 30, 2008
- 4,948
- 2,325
- 🏆 Honorable Mentions:
- 1
- Detector(s) used
- Minelab E-Trac, Equinox 600, and Tesorso compadre
- Primary Interest:
- All Treasure Hunting
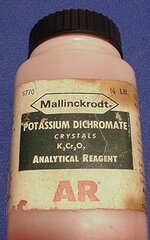
found this today at the empty lot, was under a pine tree down about 6-7" my ace 250 said it was a nickel? any ideas? My pinpointer went off at about a good inch away, BTW it is about 1.5 inches long.










 nty
nty