itzyoboyandrew
Sr. Member
- May 13, 2015
- 492
- 422
- Primary Interest:
- All Treasure Hunting
Find this alot while metal detecting.... i live south east georgia (about an hour of savannah) so go figure how it got here... i find it randomly/rather deep (6+inches) mainly in a lower lying area of our yard, theres no rocks around them... so i dont think it was in a drive way or anything... I would go with jasper... but doesnt jasper usually have stripes and stuff through the,? this is solid red.... if it is jasper does the bright red effect the value of it?
theres 2 types of have, brownish/tan color and bright red... i took pictures with water on them aswell to show there vibrance.





theres 2 types of have, brownish/tan color and bright red... i took pictures with water on them aswell to show there vibrance.








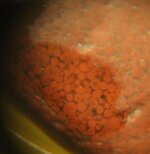


 The magnified piece does not appear to be one of the stones you first showed unless its gone thru a tumbler.
The magnified piece does not appear to be one of the stones you first showed unless its gone thru a tumbler.
