DizzyDigger
Gold Member
- Dec 9, 2012
- 6,495
- 13,283
- Detector(s) used
- Nokta FoRs Gold, a Gold Cube, 2 Keene Sluices and Lord only knows how many pans....not to mention a load of other gear my wife still doesn't know about!
- Primary Interest:
- Prospecting
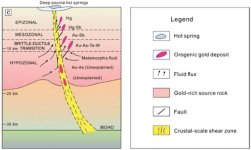
Saw this article on a site I visit, and though Y'all might find it interesting....

 phys.org
phys.org

Thermodynamic model identifies how gold reaches Earth's surface
A research team including a University of Michigan scientist has discovered a new gold-sulfur complex that helps researchers understand how gold deposits are formed.
Upvote
8