Hawkins
Greenie
- Mar 9, 2014
- 18
- 12
- Detector(s) used
- Fisher 1280-X, Excalibur 1000,
Treasure Hunter Aqua Vision Pro
- Primary Interest:
- Shipwrecks
- Thread starter
- #21
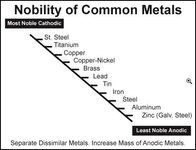
Hi Gator, I think I mentioned to Red that I wanted to use a stainless steel plate, would that work well? Another question is. . . Which items gets the positive, I think I saw on youtube that the positive gets the steel and the negative gets the anchor?
Is that wrong or is it opposite? By the way thanks for posting gator, I have been impressed with your posts and I think I'm in your area. I recently purchased a house in Vero Beach, for the purpose of exploring the 1715 beaches and possibly lease from brent.
I might have a couple investors and patient money.
Is that wrong or is it opposite? By the way thanks for posting gator, I have been impressed with your posts and I think I'm in your area. I recently purchased a house in Vero Beach, for the purpose of exploring the 1715 beaches and possibly lease from brent.
I might have a couple investors and patient money.