You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
fluxes for melt stages
- Thread starter epidote
- Start date
aussco999
Jr. Member
Hey epidote:
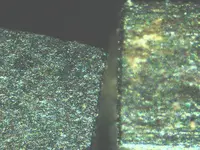
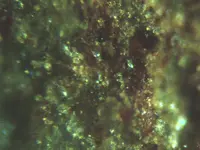
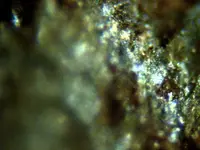
Some very interesting mineral photos and they diffidently warrant a closer "look-see". But, unless I completely misunderstood your intentions, if you are thinking about going to a direct melt/smelt to extract the precious metals from these minerals, it isn't going to happen.
The main purpose of a flux is to improve the flow of the melt and in some cases, to slag off small amounts of impurities. If the bulk of your melt is precious metals, there is a "verifiable" flux used by the U.S. Mint to oxidies off the contaminates. But, if the bulk of your melt is a base metal, there is no flux to slag off the precious metals.
At high temperatures, all the metals will mix or alloy together, inhibiting any form of seperation. You might be able to use a collector like lead (Pb) or bismuth (Bi), as done in a fire assay, but with bulk iron melts that is seldom economically fesible. And then, there's the sulfur problem.
Speaking of bulk melts, how much of this material do you have? There's always a way to extract precious metals from an ore, but I don't think direct pyrometallurgy is the answer in this case. Give us some more info on your ore and we should be able to come up with a better answer.
Good luck,
John
Some very interesting mineral photos and they diffidently warrant a closer "look-see". But, unless I completely misunderstood your intentions, if you are thinking about going to a direct melt/smelt to extract the precious metals from these minerals, it isn't going to happen.
The main purpose of a flux is to improve the flow of the melt and in some cases, to slag off small amounts of impurities. If the bulk of your melt is precious metals, there is a "verifiable" flux used by the U.S. Mint to oxidies off the contaminates. But, if the bulk of your melt is a base metal, there is no flux to slag off the precious metals.
At high temperatures, all the metals will mix or alloy together, inhibiting any form of seperation. You might be able to use a collector like lead (Pb) or bismuth (Bi), as done in a fire assay, but with bulk iron melts that is seldom economically fesible. And then, there's the sulfur problem.
Speaking of bulk melts, how much of this material do you have? There's always a way to extract precious metals from an ore, but I don't think direct pyrometallurgy is the answer in this case. Give us some more info on your ore and we should be able to come up with a better answer.
Good luck,
John
epidote
Tenderfoot
- Joined
- Mar 26, 2012
- Messages
- 6
- Reaction score
- 0
- Golden Thread
- 0
- Primary Interest:
- All Treasure Hunting
- #3
Thread Owner
reply to thread
Eventually a vehicle load. I really prefer to start with melting the Ore due to simplicity and convience of having a useable product that someone else can reduce at a industrial station. The dark cubes are probably Silver Titanium mostly but the cubes replicate for the PGM also. A few cubes dont move to a magnet. Thanks for replying.
Eventually a vehicle load. I really prefer to start with melting the Ore due to simplicity and convience of having a useable product that someone else can reduce at a industrial station. The dark cubes are probably Silver Titanium mostly but the cubes replicate for the PGM also. A few cubes dont move to a magnet. Thanks for replying.
aussco999
Jr. Member
Hey epidote:
Well, a vehicle load of this mineral should keep you busy over the weekend, but if you toss it all in the furnance and melt it down into a big lump, I still have serious doubts it will be useful to anyone.
Just a couple side notes:
I was not aware the silver and titanium associated together as a mineral, and couldn't find any reference to the fact. I know Mother Nature can come up with some strange combinations, but could you point me in the right direction where you got that info.
With safety in mind, if your ore really contains what is called Ruby Silver, as either a proustite or pyrargyrite, be aware these minerals contain antimony and arsenic, which can cause serious health problems when heated. Be careful.
And finally, have you checked the chromite content of your ore. The saying goes for hardrock, no chromite, no PGMs.
Keep us informed of your progress and good luck.
John
Well, a vehicle load of this mineral should keep you busy over the weekend, but if you toss it all in the furnance and melt it down into a big lump, I still have serious doubts it will be useful to anyone.
Just a couple side notes:
I was not aware the silver and titanium associated together as a mineral, and couldn't find any reference to the fact. I know Mother Nature can come up with some strange combinations, but could you point me in the right direction where you got that info.
With safety in mind, if your ore really contains what is called Ruby Silver, as either a proustite or pyrargyrite, be aware these minerals contain antimony and arsenic, which can cause serious health problems when heated. Be careful.
And finally, have you checked the chromite content of your ore. The saying goes for hardrock, no chromite, no PGMs.
Keep us informed of your progress and good luck.
John
epidote
Tenderfoot
- Joined
- Mar 26, 2012
- Messages
- 6
- Reaction score
- 0
- Golden Thread
- 0
- Primary Interest:
- All Treasure Hunting
- #5
Thread Owner
 In my situation nature was a certain plasma group. I recovered a Zinc Sulfur chunk having the vesic
In my situation nature was a certain plasma group. I recovered a Zinc Sulfur chunk having the vesic
 le pattern of Retroecoglite. The forming was around 40 miles deep; it was transparent with minor brown coloring. The other samples
le pattern of Retroecoglite. The forming was around 40 miles deep; it was transparent with minor brown coloring. The other samplescontained abundant Buckminster discs. The evidence proves plasmal migration and Ocean water as the source. I hope the
photos show the Black Silver as I am labeling it. The Tracyte Phono flow had abundant Aluminum (different of colors) and
dark red AlO. Academics is another world from me. The Black could be any number of elements. The volcanic flow was
all crystal; some hydrated and probably had acid that helped form rare Ore.


Last edited:
aussco999
Jr. Member
Hey epidote:
Please overlook the fact that I'm not familiar with some of your explanatory words, such as; retroecoglite, buckminster discs, tracyte phono and plasmal migration, so I'm guessing you are using colloquial terminology to describe the geology of your ore. And, now that you have added zinc sulfide and black silver ( ) to the smorgasbord mineral mix, I’m even more positive that a direct smelt/melt will not provide you a usable or salable product.
) to the smorgasbord mineral mix, I’m even more positive that a direct smelt/melt will not provide you a usable or salable product.
Because I don’t have a good enough “geologist eye” to look at a photo and know the exact mineral composition, I’m suggesting your next step should be a mass-spectrometer (55 or 70 elements) analysis, so you won’t be guessing what is the best precious metal extraction method for your ore.
Let us know what you find, and good luck.
John
Please overlook the fact that I'm not familiar with some of your explanatory words, such as; retroecoglite, buckminster discs, tracyte phono and plasmal migration, so I'm guessing you are using colloquial terminology to describe the geology of your ore. And, now that you have added zinc sulfide and black silver (
 ) to the smorgasbord mineral mix, I’m even more positive that a direct smelt/melt will not provide you a usable or salable product.
) to the smorgasbord mineral mix, I’m even more positive that a direct smelt/melt will not provide you a usable or salable product.Because I don’t have a good enough “geologist eye” to look at a photo and know the exact mineral composition, I’m suggesting your next step should be a mass-spectrometer (55 or 70 elements) analysis, so you won’t be guessing what is the best precious metal extraction method for your ore.
Let us know what you find, and good luck.
John
Similar threads
- Suggestion
- Replies
- 4
- Views
- 631
- Suggestion
- Replies
- 2
- Views
- 740
Users who are viewing this thread
Total: 1 (members: 0, guests: 1)