Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
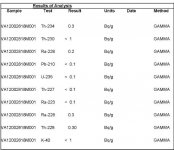
can any one read this radium test?
- Thread starter hmmm
- Start date
arizau
Silver Member
- May 2, 2014
- 2,518
- 3,947
- Detector(s) used
- Beach High Banker, Sweep Jig, Whippet Dry Washer, Lobo ST, 1/2 width 2 tray Gold Cube, numerous pans, rocker box, and home made fluid bed and stream sluices.
- Primary Interest:
- Prospecting
What you have is an analysis for several different isotopes of Thorium, Radium, and one each of Uranium, Lead and Potassium. The units are https://en.wikipedia.org/wiki/Becquerel/gram. What all that means in laymans terms I have no clue.
SunshineMiner
Full Member
- Jun 2, 2014
- 230
- 252
- Detector(s) used
- Garrett Infinium LS
- Primary Interest:
- Prospecting
arizau - I googled those as well, and even what the bq/g meant. Becquerel per gram. Becquerel is a measure of radioactive decay. So to me it almost reads that its decaying at .3% of a gram? or is it .3% of a gram of each isotope is present in the sample.
Staying tuned!
Staying tuned!
Capt Nemo
Bronze Member
I think it's number of disintegrations per second per gram. So for the Thorium 234 you have 0.3 atoms disintegrating per second in a 1 gram sample.
Some of the nuclear/particle physics units only make sense to those in the field.
An intellectual is a person that has been educated beyond his intelligence.
Some of the nuclear/particle physics units only make sense to those in the field.
An intellectual is a person that has been educated beyond his intelligence.
Bq per gram? how do you weigh an atom that is disintegrating?
may find something on describing 'units' here;
Facts and Information about Radiation Exposure - The Energy Collective
convert Becquerel to Picocuries, the units used like for safety limits,
all I could find was 1 pCi = 0.037 Bq online converter
https://www.atsdr.cdc.gov/phs/phs.asp?id=789&tid=154
The EPA has set a drinking water limit of 5 picocuries per liter (5 pCi/L) for radium-226 and radium-228 (combined).
The EPA has set a soil concentration limit for radium-226 in uranium and thorium mill tailings
of 5 picocuries per gram in the first 15 centimeters of soil and 15 picocuries per gram in deeper soil.
may find something on describing 'units' here;
Facts and Information about Radiation Exposure - The Energy Collective
convert Becquerel to Picocuries, the units used like for safety limits,
all I could find was 1 pCi = 0.037 Bq online converter
https://www.atsdr.cdc.gov/phs/phs.asp?id=789&tid=154
The EPA has set a drinking water limit of 5 picocuries per liter (5 pCi/L) for radium-226 and radium-228 (combined).
The EPA has set a soil concentration limit for radium-226 in uranium and thorium mill tailings
of 5 picocuries per gram in the first 15 centimeters of soil and 15 picocuries per gram in deeper soil.
Last edited:
SunshineMiner
Full Member
- Jun 2, 2014
- 230
- 252
- Detector(s) used
- Garrett Infinium LS
- Primary Interest:
- Prospecting
I was kind of confused about that. Considering I have 0 knowledge in this field.
winners - usually something that is??/g is per gram or w/e during measuring units correct? As someone who has 0 knowledge in the field, and just googling things, it showed a calculator for becquerels per gram for a calculation. SO that didnt help. https://www.calculand.com/unit-converter/?gruppe=Specific+Activity&einheit=Becquerel+per+gram+[Bq/g] I had the bq/g stuck in my head as becquerels per gram. Reading a bit more and Capt Nemos explanation in terms of disintegration's per gram is helpful.
Reading this gave me a little more insight: https://ieer.org/resource/classroom/measuring-radiation-terminology/
3rd paragraph of measuring radioactivity.
That and Capt Nemos response really helped. Thanks guys.
winners - usually something that is??/g is per gram or w/e during measuring units correct? As someone who has 0 knowledge in the field, and just googling things, it showed a calculator for becquerels per gram for a calculation. SO that didnt help. https://www.calculand.com/unit-converter/?gruppe=Specific+Activity&einheit=Becquerel+per+gram+[Bq/g] I had the bq/g stuck in my head as becquerels per gram. Reading a bit more and Capt Nemos explanation in terms of disintegration's per gram is helpful.
Reading this gave me a little more insight: https://ieer.org/resource/classroom/measuring-radiation-terminology/
3rd paragraph of measuring radioactivity.
That and Capt Nemos response really helped. Thanks guys.
hmmm
Hero Member
- Jun 9, 2007
- 830
- 95
- Primary Interest:
- All Treasure Hunting
- Thread starter
- #7
thanks guys i HAVE A GREEN CLAY THAT HAS THIS TEST AND I WANT TO MAKE SURE ITS SAFE. we are going to sell it as a cleaning product.
, i may have it figured out , I think it is just the "standered detection limit" meaning it has no radioactive elements. below is want i got from the testing company..
"I also attached our standard detection limits for each of the parameters of interest. We may be able to lower the detection limits, however this depends on the nature of the sample."
Th-234: 0.2 Bq/g
Th-230: 1 Bq/g
Ra-226: 0.2 Bq/g
Pb-210: 0.2 Bq/g
U-235: 0.2 Bq/g
Th-227: 0.2 Bq/g
Ra-223: 0.2 Bq/g
Ra-228: 0.2 Bq/g
Th-228: 0.2 Bq/g
K-40: 1 Bq/g
, i may have it figured out , I think it is just the "standered detection limit" meaning it has no radioactive elements. below is want i got from the testing company..
"I also attached our standard detection limits for each of the parameters of interest. We may be able to lower the detection limits, however this depends on the nature of the sample."
Th-234: 0.2 Bq/g
Th-230: 1 Bq/g
Ra-226: 0.2 Bq/g
Pb-210: 0.2 Bq/g
U-235: 0.2 Bq/g
Th-227: 0.2 Bq/g
Ra-223: 0.2 Bq/g
Ra-228: 0.2 Bq/g
Th-228: 0.2 Bq/g
K-40: 1 Bq/g
hawkeye39
Jr. Member
- May 12, 2013
- 37
- 49
- Detector(s) used
- TDI SL
Whites GMT
GB2
- Primary Interest:
- Prospecting
Looks to me that the 3 elements that register 0.3 exceed the 0.2 limit and you would have to reduce this somehow to make it acceptable. Just a guess. Why don't you get the information you need from the company doing the analysis?
Also, the test result shows you do have radioactive elements IMO.
Also, the test result shows you do have radioactive elements IMO.
Last edited:
those are just the detection limits of the machine they are using for the gamma spectrometry test.
while that test may be acceptable for soil maybe household use, if it was for cosmetics it may need a more accurate measurement.
must be somewhere to find standards like USDA or EPA or even MSDS-OSHA
doesn't seem to be very high levels, its not large bulk stored under your house like soil concentrations
looks like you are just under or right at the lower end if they were used as fill.
might do a chemical analysis or XRF for like a MSDS ie. quartz, silicon oxides, talc, bentonite...
you could put a disclaimer to not let it dry out and create dust or breath any dust.
This material is intended to be used as a cleaning
ingredient and is exempt from Toxic Substances Control
Act (TSCA) regulation (40 CFR 710) when used as such.
Do not use for other purposes.
while that test may be acceptable for soil maybe household use, if it was for cosmetics it may need a more accurate measurement.
must be somewhere to find standards like USDA or EPA or even MSDS-OSHA
doesn't seem to be very high levels, its not large bulk stored under your house like soil concentrations
looks like you are just under or right at the lower end if they were used as fill.
might do a chemical analysis or XRF for like a MSDS ie. quartz, silicon oxides, talc, bentonite...
you could put a disclaimer to not let it dry out and create dust or breath any dust.
This material is intended to be used as a cleaning
ingredient and is exempt from Toxic Substances Control
Act (TSCA) regulation (40 CFR 710) when used as such.
Do not use for other purposes.
Last edited:
D
drakesmen
Guest
the values are only slightly above the lower detection level (RDL) of the method used. Elevated or anomalous values are generally 1 or 2 orders of magnitude greater than background levels. Therefore I think they are quite low values, and not elevated, even though some are detectable.
ecmjamsit
Hero Member
- Dec 2, 2007
- 873
- 1,062
- Detector(s) used
- Whites Goldmaster GMT, GMII,Whites Sierra Super Trac, Ace250, Teknetics Gamma 6000, Whites Pinpointer,Garrett Pro Pointer II
- Primary Interest:
- Metal Detecting
It is how many gamma rays are emitted per second from a 1 gram sample. Alpha particles are stopped by paper, beta particles are stopped by aluminum foil. Gamma particles are stopped by lead!
Sounds like this stuff will be harmful if ingested, or inhaled. I inhaled some radioactive beryllium, the results are not good. My dad died from uranium exposure.
I guess you have to make sure the material passes federal law standards.
Sounds like this stuff will be harmful if ingested, or inhaled. I inhaled some radioactive beryllium, the results are not good. My dad died from uranium exposure.
I guess you have to make sure the material passes federal law standards.
D
drakesmen
Guest
It is how many gamma rays are emitted per second from a 1 gram sample. Alpha particles are stopped by paper, beta particles are stopped by aluminum foil. Gamma particles are stopped by lead!
Sounds like this stuff will be harmful if ingested, or inhaled. I inhaled some radioactive beryllium, the results are not good. My dad died from uranium exposure.
I guess you have to make sure the material passes federal law standards.
Thats not good, where the levels low?
Top Member Reactions
-
 3428
3428 -
 2012
2012 -
 1950
1950 -
 1157
1157 -
 1098
1098 -
 911
911 -
 807
807 -
 799
799 -
 798
798 -
 788
788 -
 746
746 -
 533
533 -
 477
477 -
 472
472 -
 444
444 -
 422
422 -
 419
419 -
 416
416 -
E
415
-
 388
388
Users who are viewing this thread
Total: 2 (members: 0, guests: 2)